A Hysterectomy is Not Your Only Option
Sonata is an incisionless fibroid treatment that leaves the uterus intact.
Sonata Treatment
The Sonata Treatment is an incisionless solution clinically proven to reduce fibroid symptoms including heavy menstrual bleeding. This treatment can address a wide range of fibroid types and sizes. Multiple fibroids can be treated during a single procedure.
Clinical Results
Women were surveyed 12 months after their Sonata Treatment and reported the following results:*
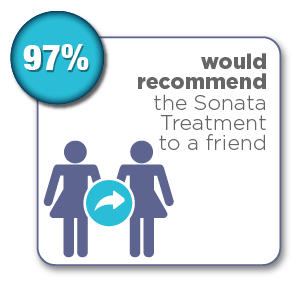
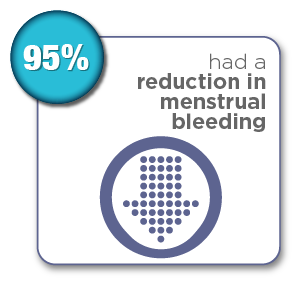
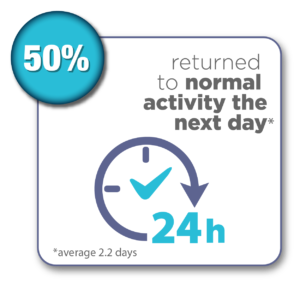
*Chudnoff S, Guido R, Roy K, Levine D, Mihalov L, Garza-Leal JG. Ultrasound-Guided Transcervical Ablation of Uterine Leiomyomas: The SONATA Trial. Obstet Gynecol. 2019 Jan; 133(1): 13-22
Sonata Success Stories
Click on an image below to learn how these women got back to enjoying life by choosing the Sonata Treatment to reduce their uterine fibroid-related symptoms
Safety Information | Impressum | Terms of Use | Careers | Contact Us |
Privacy Notice | Cookie Notice | Do not Sell/Share my Personal Information |
Limit the Use of My Sensitive Personal Information
Gynesonics, Inc. | 600 Chesapeake Drive | Redwood City, CA 94063
Copyright 2024 Gynesonics | WS 05195-001 Rev H
